42 lewis symbols
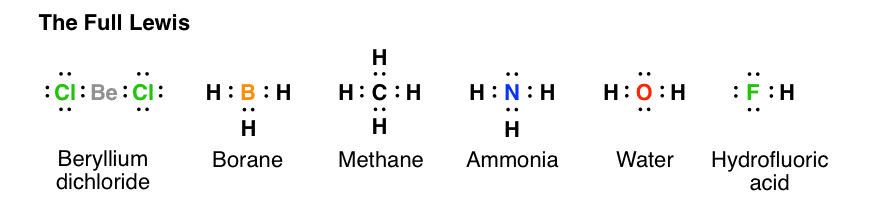
7.3 Lewis Symbols and Structures - Chemistry A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 1 shows the Lewis symbols for the elements of the third period of the periodic table. Figure 1. Lewis symbols illustrating the number of valence electrons for each element in the third period of the periodic table. Lewis Symbol - Purdue University Lewis Symbol. Lewis symbol: the symbol for an element showing a dot for each valence electron in the element or ion.
4.4 Lewis Symbols and Structures - General Chemistry 1 & 2 A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 1 shows the Lewis symbols for the elements of the third period of the periodic table. Figure 1. Lewis symbols illustrating the number of valence electrons for each element in the third period of the periodic table.

Lewis symbols
Lewis Symbols and the Octet Rule | Chemistry | JoVE A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons. For example, sodium has one valence electron; so one dot is drawn around the symbol Na. For main group elements, the number of valence electrons is indicated by a lettered group number in the periodic table. Lewis Symbols and Structures (4.4) - Chemistry 110 A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 4.9 shows the Lewis symbols for the elements of the third period of the periodic table. Figure 4.9 Lewis symbols illustrating the number of valence electrons for each element in the third period of the periodic table. lewis symbols - Madura English-Sinhala Dictionary Madura English-Sinhala Dictionary contains over 230,000 definitions. Include glossaries of technical terms from medicine, science, law, engineering, accounts, arts and many other sources. This facilitates use as thesaurus. Translate from English to Sinhala and vice versa. Can use wildcards to increase the flexibility of search.
Lewis symbols. Answered: (a) Using Lewis symbols, diagram the… | bartleby Q: Using Lewis symbols and Lewis structures, diagram the formation of SiCl4 from Si and Cl atoms,… A: Click to see the answer Q: Draw a Born-Haber cycle for the formation of LiF(s) from its elements. Danube Odyssey, Part 11 - By LEWIS NOLAN Index to Photos / Page Updated Jan. 19, 2008 - (More than 200 additional photos taken on the Nolans' two-week cruise through parts of Austria, Germany, Hungary and Slovakia are posted in several Lewis Nolan albums at , a website that requires sign-in) . By LEWIS NOLAN. Return to Nolan Travels Home Page . Nov. 24, 2007, Saturday - In Nuremberg Lewis Symbols - Chemistry LibreTexts A Lewis Symbol is constructed by placing dots representing electrons in the outer energy around the symbol for the element. For many common elements, the number of dots corresponds to the element's group number. Below are Lewis Symbols for various elements. Notice the correspondence to each element's group number. Lewis Diagrams for Molecules Lewis Symbols and Structures - Chemistry 2e A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: (Figure) shows the Lewis symbols for the elements of the third period of the periodic table. Lewis symbols illustrating the number of valence electrons for each element in the third period of the periodic table.
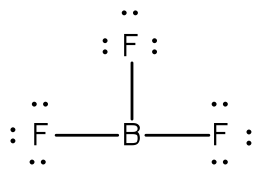
Lewis Symbols | Chemical Bonding - Nigerian Scholars A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: The figure below shows the Lewis symbols for the elements of the third period of the periodic table. Lewis symbols illustrating the number of valence electrons for each element in the third period of the periodic table. 4.4 Lewis Symbols and Structures - Chemistry: Atoms First 2e - OpenStax We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 4.10 shows the Lewis symbols for the elements of the third period of the periodic table. Drawing Lewis Dot Symbols or Electron Dot Diagrams - YouTube Drawing Lewis Dot Symbols or Electron Dot Diagrams is an important skill in understanding molecular geometry and ionic crystals. It also helps with understan... Lewis Symbols - Chemical Bonding and Molecular Structure G.N. Lewis introduced simple symbols to denote the valence electrons in an atom. The outer shell electrons are shown as dots surrounding the symbol of the atom. These symbols are known as Lewis symbols are electron dot symbols. Significance The number of dots around the symbol give the number of electrons present in the outermost shell.
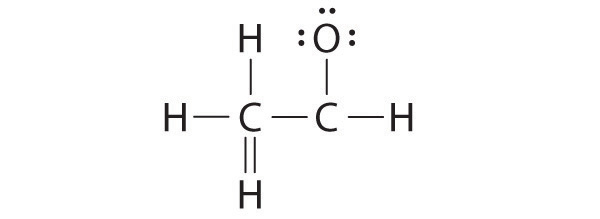
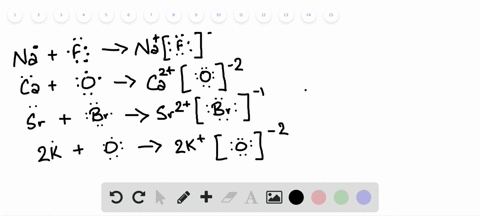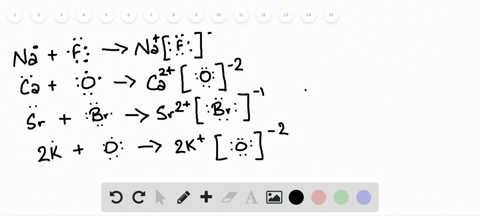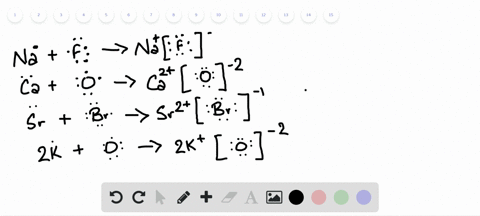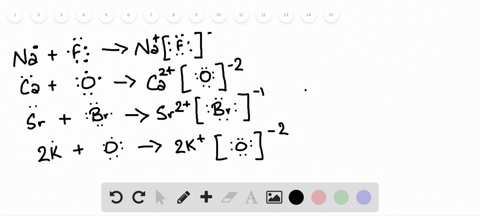
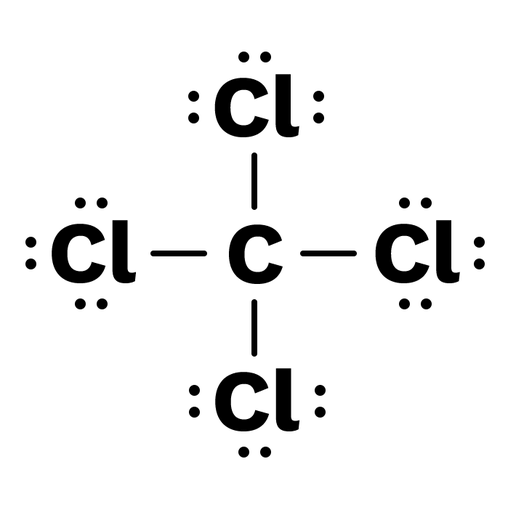
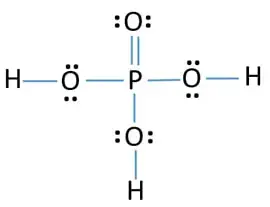
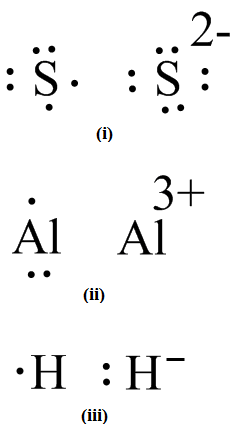
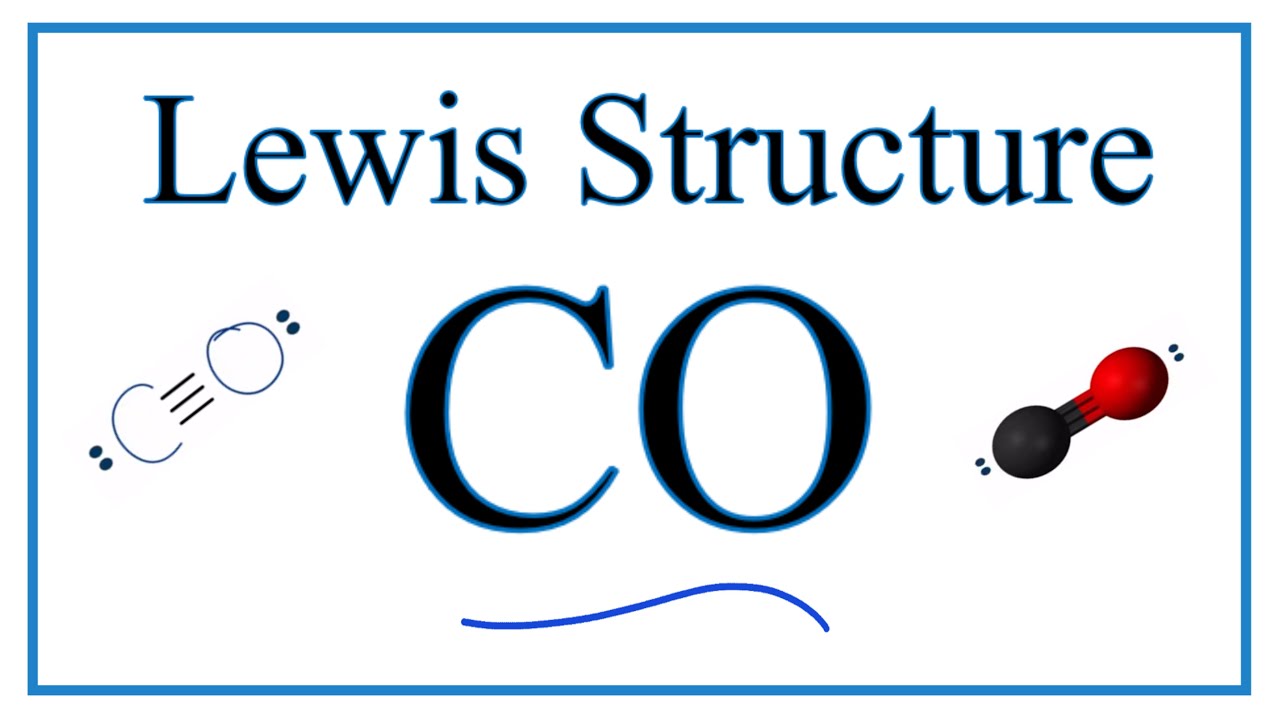
Lewis Symbols and Structures - Chemistry Lewis symbols illustrating the number of valence electrons for each element in the third period of the periodic table. Lewis symbols can also be used to illustrate the formation of cations from atoms, as shown here for sodium and calcium: Likewise, they can be used to show the formation of anions from atoms, as shown here for chlorine and sulfur: Lewis Structure Definition and Example - ThoughtCo A Lewis structure is based on the concept of the octet rule, in which atoms share electrons so that each atom has eight electrons in its outer shell. As an example, an oxygen atom has six electrons in its outer shell. In a Lewis structure, these six dots are arranged so that an atom has two lone pairs and two single electrons. 7.3 Lewis Symbols and Structures - Chemistry A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 1 shows the Lewis symbols for the elements of the third period of the periodic table. Figure 1. Lewis symbols illustrating the number of valence electrons for each element in the third period of the periodic table. Lewis structure - Wikipedia Lewis structures show each atom and its position in the structure of the molecule using its chemical symbol. Lines are drawn between atoms that are bonded to one another (pairs of dots can be used instead of lines). Excess electrons that form lone pairs are represented as pairs of dots, and are placed next to the atoms.
Lewis structure and octet rule Lewis structure, also called electron-dot structure, is a structural formula in which electrons are represented by dots; two dots between two atoms represent a ...1 page
Lewis Structure | Encyclopedia.com In a Lewis symbol, the symbol of the element represents the nucleus and the core electrons. Dots placed around the symbol of the element represent the valence electrons. Lone pair— A pair of electrons located around an atom but not shared with another atom is called a lone pair, an unshared pair or a non-bonding pair.
Lewis Structures: Learn How to Draw Lewis Structures | Albert.io This type of Lewis dot structure is represented by an atomic symbol and a series of dots. See the following examples for how to draw Lewis dot structures for common atoms involved in covalent bonding. Example 1. Draw the Lewis Dot Structure for the Hydrogen atom. Since Hydrogen is in Group I it has one (1) valence electron in its shell. Example 2.
The Lewis - A symbol in Freemasonry. A Lewis is an operative tool, Masonic symbol, or young man adopted by a Lodge. A lewis is an instrument in operative masonry. It is a cramp iron which is inserted into a cavity prepared for that purpose in any large stone, so as to give attachment to a pulley and hook whereby the stone may be conveniently raised to any height and deposited in ...
6.01 Lewis symbols - Week 6 | Coursera These structures provide information about the types of bonds (single, double, or triple) as well as the connectivity of atoms. By knowing the Lewis structure, we can also predict the three-dimensional geometry of an individual molecule. 6.01 Lewis symbols 4:36. 6.02 Covalent bonds 5:19. 6.03 Electronegativity 11:21.
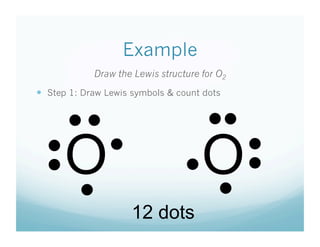
PPT - Lewis Symbols PowerPoint Presentation, free download - ID:2865110 Lewis Symbols • To help us to focus on the valence electrons - those that can participate in bonding - we use Lewis Symbols (in honor of scientist G.N. Lewis). Lewis Dot Symbols • Lewis Dot symbol (or Electron dot symbol) • Dots placed around an element's symbol represent valence electrons • Pair electrons as needed • Ions are placed in brackets with charge outside • Easily ...
Lewis Symbols | Practice Questions - organicmystery.com Lewis SymbolsPractice Questions. Lewis Symbols. We discussed in chemical bonding and Lewis symbols that Lewis dot symbols are used to represent valence electrons. Now, it's time you answered some questions on Lewis symbols. Write Lewis symbol of Mg. Write Lewis symbols of S and S 2−. The atomic number of sulphur is 16.
6.1 Lewis Symbols and Structures - Chemistry Fundamentals A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 6.1.1 shows the Lewis symbols for the elements of the third period of the periodic table. Figure 6.1.1 Lewis symbols illustrating the number of valence electrons for each element in the third period of the periodic table.
Lewis Symbols or Diagrams - Elmhurst University A Lewis Symbol consists of the element symbol surrounded by "dots" to represent the number of electrons in the outer energy level as represented by a Bohr Diagram. The number of electrons in the outer energy level is correlated by simply reading the Group number.
Lewis Symbols and Structures - chem-textbook Lewis symbols illustrating the number of valence electrons for each element in the third period of the periodic table. Lewis symbols can also be used to illustrate the formation of cations from atoms, as shown here for sodium and calcium: Likewise, they can be used to show the formation of anions from atoms, as shown here for chlorine and sulfur:
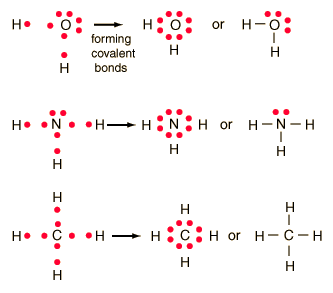
Lewis Diagrams for Compound Formation - GSU Lewis Diagrams for Compound Formation The formation of many common compounds can be visualized with the use of Lewis symbols and Lewis diagrams. In a Lewis symbol, the inner closed shells of electrons can be considered as included in chemical symbol for the element, and the outer shell or valence electrons are represented by dots.
Lewis Structures: Dot Symbols, Diagram, Examples - Embibe In a Lewis Structure, electrons are represented as "dots" surrounding the central metal atom. The central metal is denoted by using its chemical symbol from the Periodic Table. Learn Exam Concepts on Embibe In Lewis Structures, a line is used to represent the bonding electrons between two combining atoms.
lewis symbols - Madura English-Sinhala Dictionary Madura English-Sinhala Dictionary contains over 230,000 definitions. Include glossaries of technical terms from medicine, science, law, engineering, accounts, arts and many other sources. This facilitates use as thesaurus. Translate from English to Sinhala and vice versa. Can use wildcards to increase the flexibility of search.
Lewis Symbols and Structures (4.4) - Chemistry 110 A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 4.9 shows the Lewis symbols for the elements of the third period of the periodic table. Figure 4.9 Lewis symbols illustrating the number of valence electrons for each element in the third period of the periodic table.
Lewis Symbols and the Octet Rule | Chemistry | JoVE A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons. For example, sodium has one valence electron; so one dot is drawn around the symbol Na. For main group elements, the number of valence electrons is indicated by a lettered group number in the periodic table.









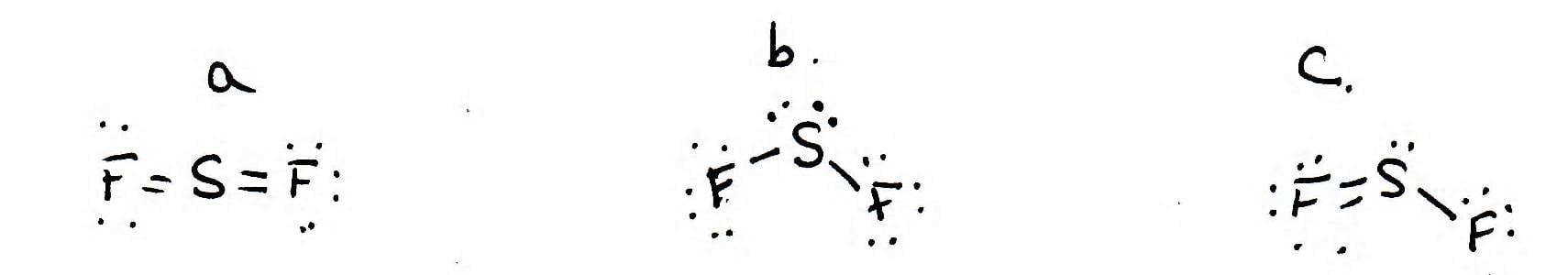











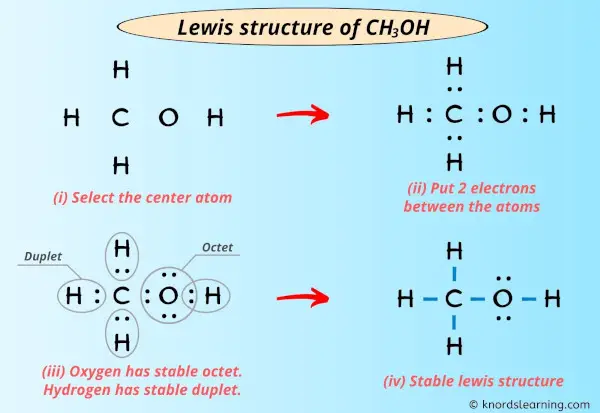

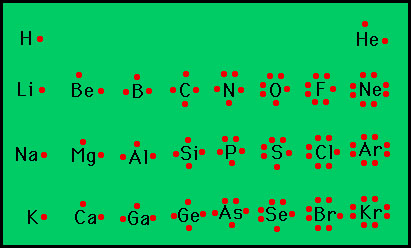

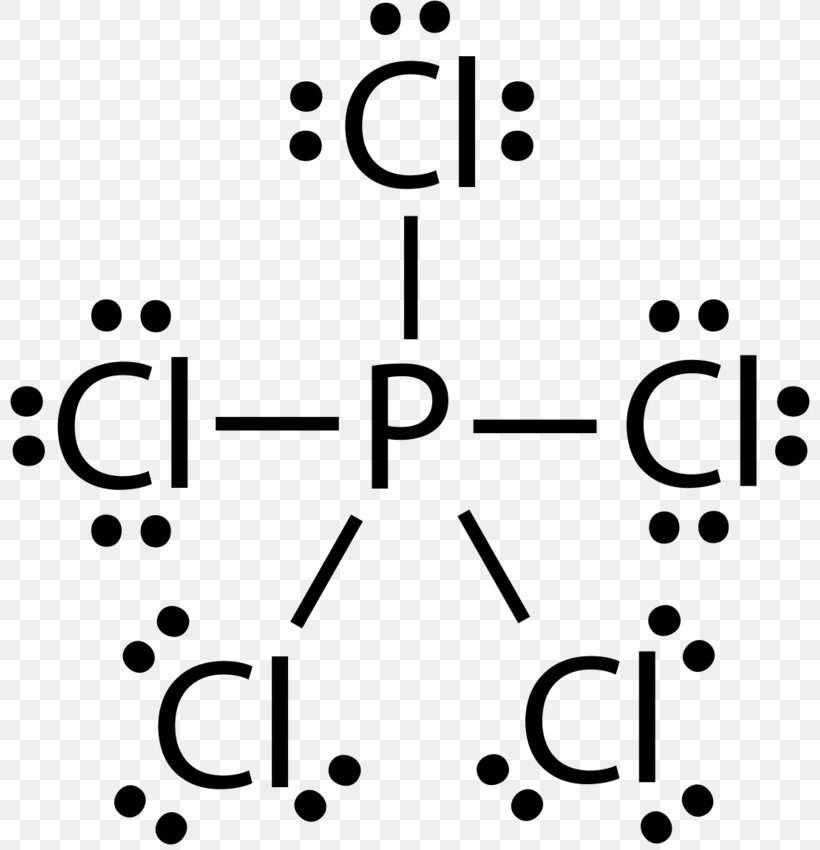
Post a Comment for "42 lewis symbols"